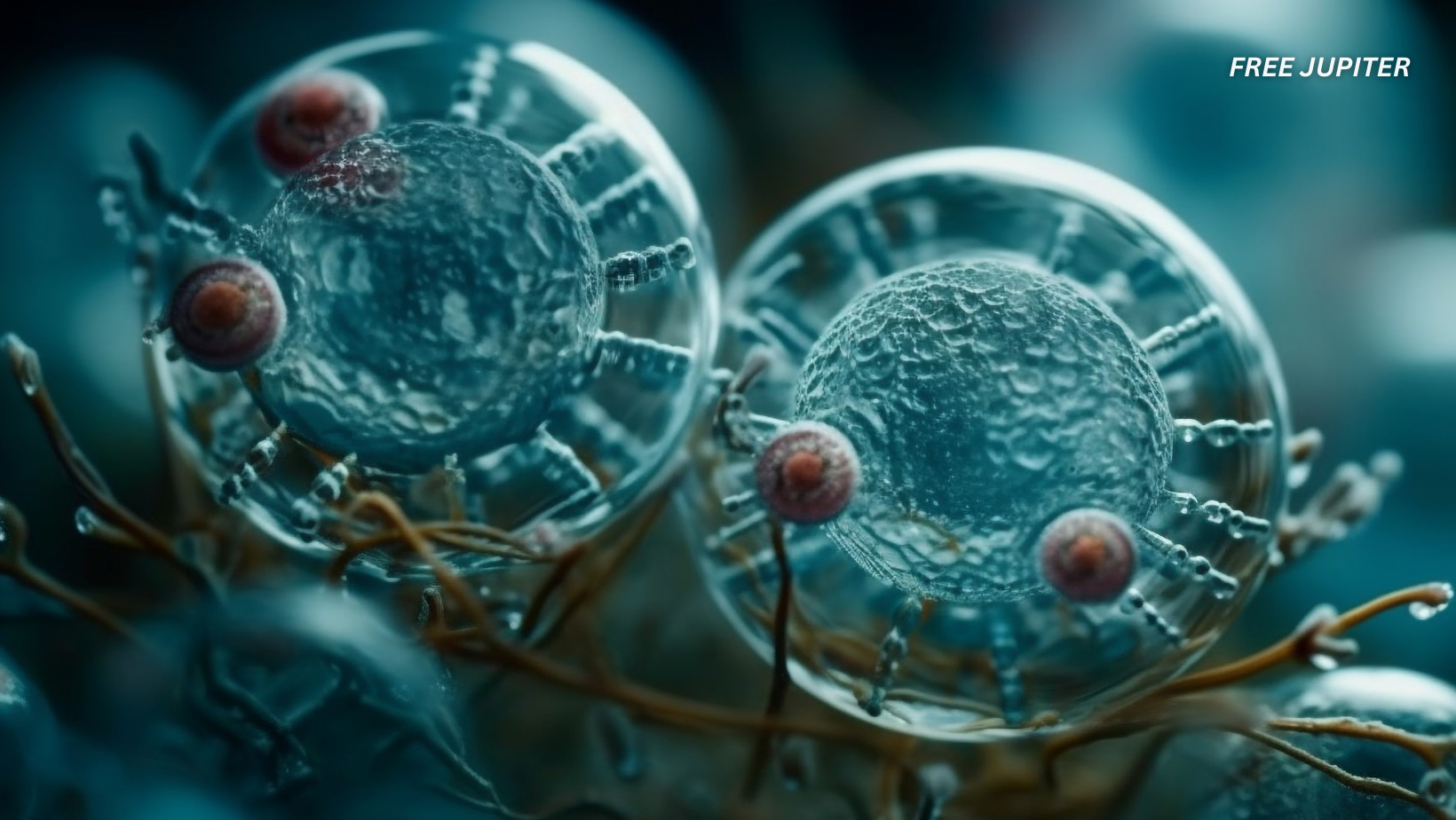
In a major medical breakthrough, scientists at the Peter Doherty Institute for Infection and Immunity in Melbourne have successfully eliminated HIV from infected human immune cells in the lab. They used a specially designed lipid nanoparticle (called LNP-2) to deliver messenger RNA (mRNA) into resting CD4+ T cells, which are the main hideout for dormant HIV. This approach forces the latent virus to reactivate, allowing it to be identified and eliminated. The findings were published in the journal Nature Communications in June 2025 and are being hailed as a potential stepping stone toward a functional cure.
This method tackles one of HIV’s biggest challenges. Traditional antiretroviral therapy (ART) suppresses viral replication but does not remove latent virus from cellular reservoirs. These dormant viral copies allow HIV to bounce back if treatment is stopped. The new mRNA approach lights up these hidden cells, giving scientists a direct way to expose and eventually destroy the virus completely.
Read Me: Many Of The Dead Sea Scrolls May Be Older Than We Thought, Experts Say
Why This Breakthrough Matters More Than Ever
HIV has remained one of the most elusive viruses to cure due to its ability to hide in long-living immune cells. These viral reservoirs act as silent saboteurs, unaffected by medication and invisible to the immune system. Even when a patient has undetectable viral loads, stopping medication causes the virus to return within weeks.
The new strategy changes this narrative. Instead of targeting only active virus replication, this approach focuses on the latent virus that hides quietly inside cells. By forcing the virus out of hiding, researchers make it vulnerable to the immune system or other therapeutic interventions. This concept of a “shock and kill” strategy has been theorized before, but using LNP-mRNA delivery makes it far more precise and effective.
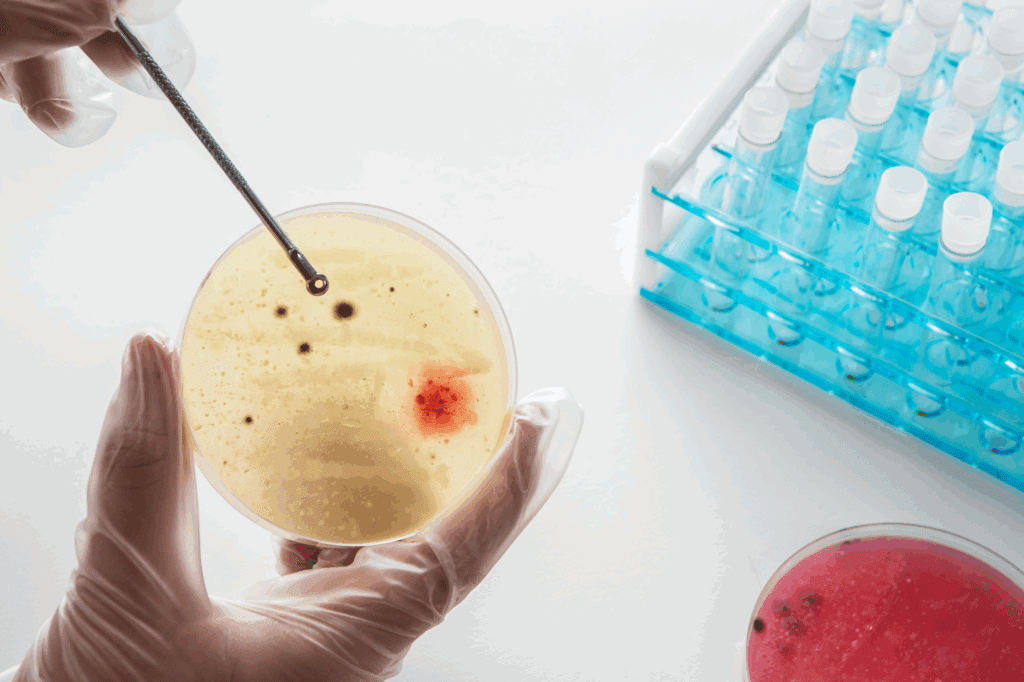
CRISPR’s Longstanding Role in HIV Research
CRISPR-Cas9 has played a crucial role in experimental HIV cures. In previous studies, CRISPR was used to cut out integrated HIV DNA from the host genome in animal models. In 2019, a combination of CRISPR-based gene editing and long-acting antiretroviral therapy cured a third of HIV-infected humanized mice. These results were promising but limited in scalability and precision.
What makes the new method revolutionary is that it works upstream of CRISPR. Instead of editing already active cells, it activates the virus in hidden cells, making them visible to CRISPR tools or immune responses. This complementary mechanism means CRISPR can now be used more accurately, reducing the risk of cutting unintended DNA sequences.
The synergy between these tools strengthens the case for a multi-pronged approach to HIV elimination. Each tool plays a unique role, and together they provide a much more effective framework than any one method alone.
Where mRNA Meets CRISPR: The Perfect Storm Against HIV
The integration of mRNA delivery with CRISPR gene editing could be the key to finally dismantling the HIV reservoir. mRNA is used to encode specific proteins that trigger the reactivation of latent HIV. Once the virus is reawakened, CRISPR can then locate and remove the infected DNA with greater precision.
What sets this research apart is that it uses a lipid nanoparticle vehicle that has already been approved for vaccine delivery, such as those used in COVID-19 vaccines. This means that the delivery mechanism is not only effective but potentially safe for human trials. The approach sidesteps some of the immune reactions that previously plagued gene therapy research.
The researchers describe this dual-action method as a “seek and destroy” technique, with mRNA being the locator and CRISPR being the eliminator. It is a smart, targeted approach that limits collateral damage and enhances therapeutic potential.
Read Me: Experts Explain Shocking Simulation That Reveals What Eating One Meal a Day Does to Your Body
Safety Concerns and Why Caution Still Rules
Despite this enormous progress, experts emphasize that safety remains a top priority. Exposing latent HIV does not guarantee full elimination. The body’s immune system may not be able to clear all reactivated cells on its own. Combining this method with immunotherapy or CRISPR might be necessary for complete eradication.
Another concern involves the specificity of delivery. HIV hides in various body compartments, including the brain, lymph nodes, and gut. Ensuring that the nanoparticle-mRNA complex reaches all these areas without triggering off-target effects is a major hurdle. While the lipid nanoparticle method has proven successful in blood-based immune cells, systemic delivery is more complex.\

Regulatory agencies will require robust safety data before approving any human trial. Researchers are already conducting extensive animal studies to better understand dosage, delivery range, and unintended impacts. Any treatment must demonstrate long-term safety, especially since the goal is to treat patients who are otherwise healthy due to antiretroviral therapy.
What This Means for the Future of HIV Treatment
This advancement does not mean an immediate end to antiretroviral therapy. For now, ART remains the gold standard for managing HIV. However, the study opens the door to long-term strategies aimed at curing the virus rather than just controlling it.
The ultimate goal is to eliminate the HIV reservoir, which would allow patients to stop ART without experiencing viral rebound. If successful in clinical trials, this could dramatically change the global HIV treatment landscape. Millions of people could eventually stop lifelong medication, reducing both healthcare costs and long-term side effects.
Human clinical trials are the next major milestone. These will begin with safety assessments, followed by small-scale efficacy trials. If results continue to show success, larger trials will follow. Realistically, widespread clinical application is still years away, but the path forward is finally clearer.
Who Will Benefit First
If approved, this approach could first be offered to patients with well-controlled HIV who are already on ART and have stable health profiles. They are most likely to benefit from reservoir elimination strategies with minimal complications. In the future, newly diagnosed patients might receive combination therapy that includes mRNA delivery and CRISPR as a frontline treatment rather than lifelong ART.
There are also implications for viral prevention. If the reservoir can be eliminated, the risk of transmission drops to near zero, even without medication. This could reduce global HIV incidence, especially in regions where access to ART is limited.
While not yet a cure, this research marks one of the most promising steps in decades. By combining the precise targeting power of CRISPR with the controlled activation capacity of mRNA, scientists are inching closer to a world where HIV can be not just managed, but truly eliminated.