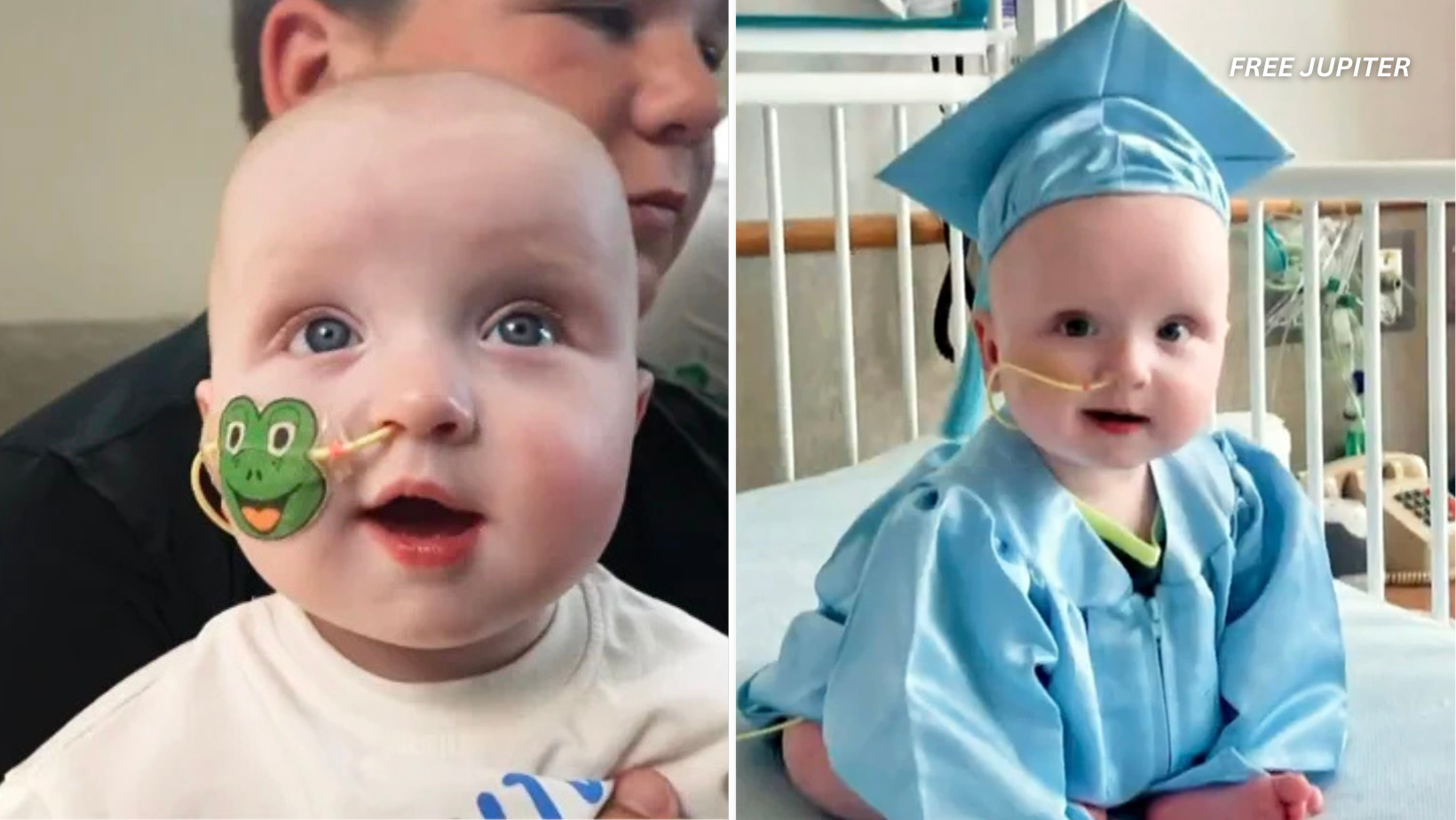
KJ Muldoon’s story is nothing short of a medical milestone. Born with an extraordinarily rare and life-threatening genetic disorder called severe carbamoyl phosphate synthetase 1 (CPS1) deficiency, KJ faced an uphill battle from the very beginning. This disorder disrupts the body’s ability to process ammonia, a toxic waste product, causing it to accumulate to dangerous levels that can lead to irreversible brain damage and death. Traditionally, treatment options for CPS1 deficiency have been limited and often involve risky liver transplants, with more than half of affected infants not surviving past early infancy.
In a groundbreaking development, a team of researchers and clinicians at the Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania’s Perelman School of Medicine harnessed the power of CRISPR gene-editing technology to create a personalized therapy tailored uniquely to KJ’s genetic makeup. This bespoke treatment, carefully designed to correct the specific mutation in KJ’s liver cells, represents the first successful use of a personalized CRISPR-based medicine administered directly to a human patient. Remarkably, the entire process—from diagnosis shortly after birth to the delivery of the therapy—was accomplished within six months, showcasing an unprecedented speed in developing individualized gene therapies.
Understanding KJ’s Rare Genetic Challenge
Shortly after birth, KJ was diagnosed with carbamoyl-phosphate synthetase 1 deficiency (CPS1D), an exceptionally rare and life-threatening metabolic disorder. This condition affects roughly one in every 1.3 million individuals and is characterized by the body’s inability to safely process and eliminate ammonia, a toxic byproduct of protein metabolism. Without effective treatment, ammonia accumulates to dangerous levels, leading to severe neurological damage and often death within the first few months of life.
KJ’s case was particularly severe, classified as the most critical variant of CPS1D. This severity meant that conventional therapies, including dietary restrictions and ammonia-scavenging drugs, were insufficient to manage his condition. He was admitted to the Children’s Hospital of Philadelphia (CHOP), where his medical team faced the daunting task of finding an effective treatment for a disease so rare that no standard protocol existed.
Read more: The First AI Platform for Breast Cancer Prediction Has Been Approved By The FDA
The Advent of Gene-Editing: CRISPR as a Game Changer
The solution came in the form of CRISPR, a cutting-edge gene-editing technology that enables precise alterations to DNA sequences. Unlike traditional gene therapies that often introduce new genetic material into the body, CRISPR works by directly correcting mutations within the genome, offering a highly personalized approach to treatment.
At seven months old, KJ became one of the first patients to receive a bespoke CRISPR-based therapy designed specifically to correct the genetic mutation causing his disorder. This treatment was developed in-house at CHOP, leveraging their advanced research capabilities and expertise in genetic medicine.
Dr. Ahrens-Nicklas, a leading physician involved in KJ’s care, emphasized the urgency and novelty of the approach: “Given the severity of KJ’s variant, we had to fast-track the development of a personalized therapy we were already exploring.”
The Road to Recovery and Discharge
Following the gene-editing treatment, KJ’s condition improved markedly. His ammonia levels decreased to safer ranges, and he began gaining weight at a healthy pace, aligning with developmental milestones typical for his age. After 307 days of intensive care and observation, KJ was deemed stable enough to leave the hospital.
To honor this achievement, the hospital staff organized a touching “graduation” ceremony. Wearing a tiny cap and gown, KJ was cheered on by doctors, nurses, and hospital personnel. Local law enforcement accompanied him on his journey home, symbolizing community support and the significance of this medical milestone.
The Broader Context: Personalized Medicine and Gene Therapy
KJ’s case exemplifies the transformative potential of personalized gene-editing therapies for treating ultra-rare genetic disorders. While gene therapies have been approved for more common conditions such as sickle cell disease and beta thalassemia—blood disorders affecting tens of thousands in the United States—tailored treatments for rare diseases like CPS1D remain largely experimental.
The ability to develop bespoke therapies within hospital settings marks a significant advancement in precision medicine. This approach allows for rapid development and deployment of treatments tailored to an individual’s unique genetic makeup, bypassing the lengthy process of pharmaceutical drug development.
Dr. Kiran Musunuru, director of the Penn Cardiovascular Institute’s Genetic and Epigenetic Origins of Disease Program, likened CRISPR’s function to a GPS system: “You can adjust the destination depending on the specific gene sequence you want to modify.”
Read more: A Swiss Company Has Created A Coffee Paste In A Tube – Perfect For Your Next Adventure
Challenges and Future Directions
Despite the promising outcome in KJ’s case, several challenges remain before such therapies become widely accessible. The complexity of gene-editing technology requires rigorous safety evaluations to prevent off-target effects and unintended genetic changes. Additionally, the high cost and technical expertise needed to develop bespoke treatments limit widespread application.
However, ongoing research is addressing these hurdles. Advances in delivery methods, such as improved viral vectors and non-viral nanoparticles, are enhancing the precision and safety of gene editing. Moreover, regulatory frameworks are evolving to accommodate personalized therapies, balancing innovation with patient safety.
Experts are hopeful that as these technologies mature, more infants born with rare genetic mutations will benefit from customized treatments, potentially transforming the prognosis for many previously untreatable conditions.
Related Advances in Gene Therapy
The success of KJ’s treatment aligns with broader progress in gene therapy. For instance, therapies for sickle cell disease have shown remarkable results, with some patients achieving long-term remission after receiving gene-modified stem cells. Similarly, beta thalassemia patients have experienced significant reductions in transfusion dependence following gene therapy.
These advances underscore the potential of gene editing not only to treat symptoms but to address the root genetic causes of disease. The ability to tailor treatments to individual genetic profiles heralds a new era in medicine, where personalized interventions can improve outcomes and quality of life.
The Human Element: Hope and Resilience
Beyond the scientific achievements, KJ’s story is a powerful reminder of the human spirit’s resilience and the profound impact of medical innovation on families. His parents and siblings now look forward to a future filled with possibilities previously unimaginable.
The medical community’s collective effort—from researchers and clinicians to hospital staff and support personnel—demonstrates the collaborative nature of modern healthcare. KJ’s journey is not just a medical milestone but a beacon of hope for countless families facing rare and devastating diseases.
Read more: A DNA Technique Is Helping To Find Women Who Deserted Their Babies
Conclusion
KJ Muldoon’s discharge from the hospital after receiving a pioneering gene-editing therapy represents a landmark moment in the treatment of rare genetic disorders. His case showcases the potential of CRISPR technology to deliver personalized, effective treatments where none existed before.
As research continues and gene-editing therapies evolve, the prospect of curing or significantly ameliorating ultra-rare diseases becomes increasingly attainable. KJ’s story offers a glimpse into a future where precision medicine transforms lives, turning once-fatal diagnoses into manageable conditions and opening new horizons for patients and their families.